Thermal graphite film is a novel thermal management material, also known as thermal graphite sheets or heat dissipation graphite film. Its primary component is carbon, featuring a unique layered hexagonal lattice structure similar to stacked graphene. This structure enables efficient heat transfer within the lattice plane. Thermal graphite film exhibits exceptionally high thermal conductivity in the horizontal plane of the sheet, far surpassing most metals. With a density of approximately 1.8~2.2 g/cm3, it is significantly lighter than metals like copper and aluminum, contributing to reduced overall equipment weight. Its thickness ranges from several micrometers to hundreds of micrometers, offering excellent flexibility. This allows it to conform to curved and irregular surfaces, adapting to the compact and complex internal spatial layouts of modern electronic devices.

The preparation of thermally conductive graphite films using polyimide (PI) films as precursors involves converting organic polymer PI into inorganic crystalline graphite through high-temperature pyrolytic rearrangement. This process comprises two core stages: carbonization and graphitization, supplemented by pre-treatment and post-treatment steps. The entire procedure relies on the molecular structural properties of PI to achieve the ordered rearrangement of carbon.
PI film serves as an ideal precursor for thermal graphite films due to two fundamental molecular properties. Its molecular chains, composed of aromatic rings like benzene and imide rings, contain over 70% carbon by mass. Pyrolysis preserves substantial carbon skeletons, providing ample carbon sources for graphitization. PI films prepared via processes like casting and stretching exhibit preferential molecular chain alignment parallel to the film plane. This pre-oriented structure lays the foundation for carbon atoms to form layered graphite structures along the plane during subsequent carbonization and graphitization, serving as the core prerequisite for the high in-plane thermal conductivity of the final graphite film. Moreover, PI exhibits a thermal decomposition temperature exceeding 500oC, preventing violent decomposition at medium-to-low temperatures. This allows for stable initial rearrangement of molecular chains, avoiding collapse of the carbon skeleton.
The raw PI film undergoes cleaning and drying. Some processes involve secondary stretching or thermal setting of the PI film. This further enhances the planar orientation of PI molecular chains, reduces molecular entanglement, promotes more orderly pre-alignment of the carbon skeleton, and improves the crystallinity and thermal conductivity of the final graphite film.
This is the critical step converting PI’s organic polymer structure into an inorganic carbon framework. The PI film is gradually heated in an inert atmosphere, undergoing pyrolysis, dehydrogenation, cyclization, and polycondensation reactions to ultimately form an amorphous carbon film. At this stage, the carbon film lacks distinct graphitic crystalline structure and exhibits low thermal conductivity (approximately 100~300 W/(m·K)), while retaining the planar orientation of the PI film.
This is the core step for achieving the ultra-high thermal conductivity of graphite films. The carbonized amorphous carbon film undergoes carbon atom rearrangement and crystallization at ultra-high temperatures (2000~3000oC), ultimately forming a highly ordered layered graphite crystal structure—the thermal graphite film. The extreme temperatures provide sufficient activation energy for carbon atoms to break existing cross-links in the amorphous carbon framework. Carbon atoms then rearrange along the film's plane, forming a hexagonal honeycomb lattice similar to graphene. Within the graphite layers, a large number of free electrons and phonons exist in the plane, allowing heat to be rapidly transferred through electrons and phonons. Therefore, the graphite film possesses ultra-high in-plane thermal conductivity. However, the van der Waals forces between layers are weak, hindering phonon transfer. Consequently, the thermal conductivity perpendicular to the plane is lower, resulting in thermal anisotropy.
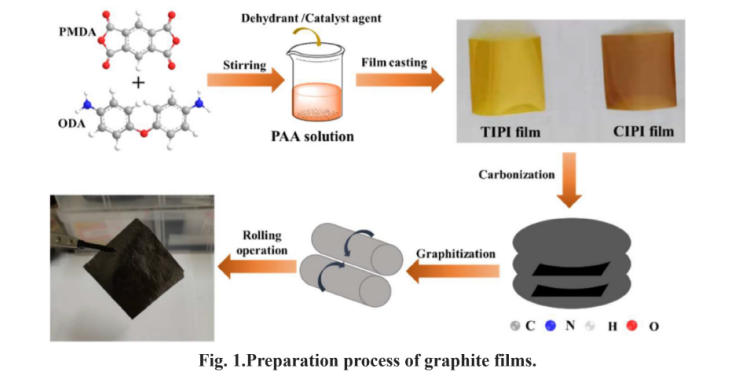
The graphitized membrane undergoes cooling, cutting, calendering, and lamination according to application requirements.
Thermal graphite film has become a critical material for electronic device heat dissipation due to its core properties: ultra-high in-plane thermal conductivity, lightweight flexibility, and anisotropic thermal conductivity. Its applications span consumer electronics, communications, new energy, industrial manufacturing, and other scenarios.
Consumer Electronics: Widely used in smartphones, tablets, laptops, and other devices to cover primary heat sources like processors, batteries, and display driver chips, aiding heat dissipation.
LED Lighting: Adhered to the substrate of LED strips or boards to facilitate heat spreading and dissipation, extending LED lifespan and maintaining luminous efficiency.
Communication Equipment: Used for heat dissipation in 5G routers, base station components, and other devices.
Wearable Devices: Leveraging its lightweight, thin, and flexible properties, it is employed for heat dissipation in smartwatches, fitness bands, and similar wearables.

Contact us to learn more about our advanced electronic chemicals and speciality polymer materials, and how they can enhance your production performances.