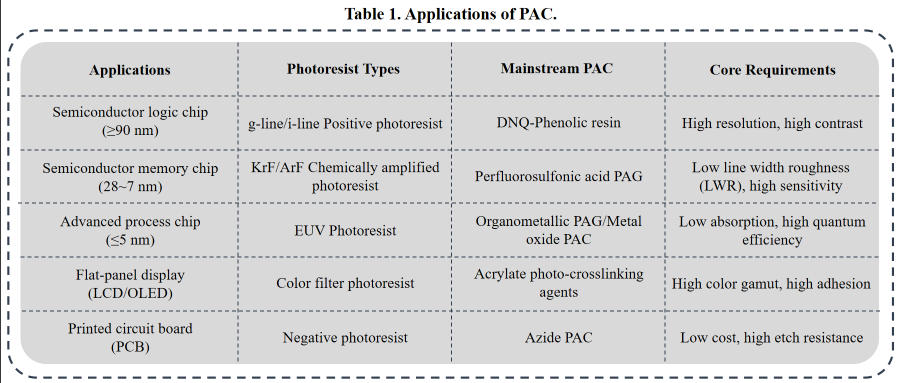
Photoactive Compounds (PAC) are a class of substances that undergo photodecomposition, cross-linking, isomerization, and other chemical reactions when exposed to light of specific wavelengths. These reactions trigger abrupt changes in the physicochemical properties of photoresist coatings, such as solubility and polarity. In photolithography, PACs play a central role in converting “light signals into chemical signals into pattern signals.” They differentiate between dissolved and undissolved regions through light exposure, ultimately forming the desired photolithographic pattern under the action of developer solution. PACs serve as the core functional component in photoresist formulations, with its properties directly determining critical process metrics such as photosensitivity, resolution, and contrast. It is one of the key materials influencing the precision and yield of semiconductor lithography processes.
Based on photoresist type and exposure wavelength, PACs exhibit significant chemical structural variations and can be categorized as follows:
In positive photoresists, PACs function by inhibiting the dissolution of the film-forming resin before exposure. After exposure, PACs decompose and release this inhibition. The most typical example is diazonaphthoquinone (DNQ) PACs. Before exposure, DNQ groups form hydrogen bonds or charge-transfer complexes with the phenolic resin, preventing its dissolution in alkaline developer solutions. After exposure, DNQ groups absorb photon energy, undergo photodecomposition, release N2, and generate an enone intermediate, which further hydrolyzes to form carboxylic acid groups. During development, these carboxylic acid groups—strongly hydrophilic—not only release the dissolution inhibition on the resin but also accelerate the dissolution of exposed resin in alkaline developer, ultimately forming a positive pattern.
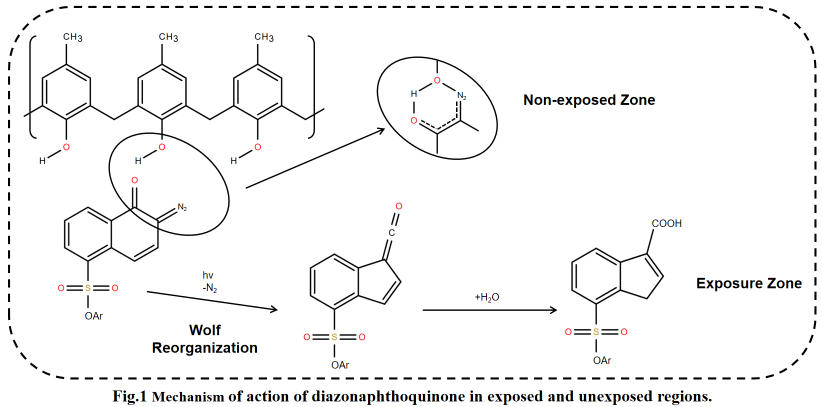
In negative photoresists, PACs function by initiating cross-linking of the film-forming resin after exposure, forming an insoluble network structure. Common types include azide-based, diazide-based, and acrylate-based PACs. Azide-based PACs contain -N3 groups that decompose upon exposure to generate highly reactive nitrogen-based radicals, initiating cross-linking reactions between resin molecules. This transforms exposed areas from soluble to insoluble. Acrylate-based PACs function as photopolymerization initiators, generating free radicals upon exposure that trigger polymerization and crosslinking of acrylate resins. They are suitable for UV-curable negative photoresists, such as PCB photoresists.
In KrF (248 nm), ArF (193 nm), and EUV (13.5 nm) photoresists, traditional DNQ-type PACs have been phased out due to excessive optical absorption coefficients and insufficient resolution. The core PAC for chemically amplified resist (CAR) is the photoacid generator (PAG), a specialized branch of PACs. Upon exposure, PAGs generate strong acids that catalyze the deprotection reaction of the film-forming resin, achieving a dissolution property transformation. EUV photoresist PACs must satisfy low absorption and high quantum efficiency. Mainstream options include metal oxide-based PACs (e.g., tin-based compounds) or organometallic PAGs, which generate acids or free radicals upon EUV photon excitation to drive pattern formation.
The performance of PACs directly determines the process window of photoresist. Core metrics include:
· Sensitivity: The minimum exposure dose (mJ/cm2) required for PACs to undergo effective photochemical reactions. Higher sensitivity enables shorter exposure times and greater efficiency in semiconductor production lines. DNQ-type PACs typically exhibit sensitivity ranging from 10 to 50 mJ/cm².
· Resolution: The smallest pattern size distinguishable by the PACs, determined by PACs molecular size and the spatial uniformity of the photoreaction. Advanced processes (e.g., below 7 nm) require PACs molecular sizes under 10 nm and photoreactions free of diffusion effects.
· Contrast (γ): Measures the solubility difference between exposed and unexposed areas. Higher contrast yields sharper pattern edges and greater lithographic precision. DNQ-based PACs typically exhibit a contrast γ exceeding 3, meeting semiconductor fine-processing demands.
· Chemical Stability: Must withstand acidic/alkaline environments and high-temperature baking during lithography without decomposition or side reactions.
As lithography technology continues to advance, demands on PACs will progressively increase. Through refined molecular structure design, diffusion of photoreaction products will be minimized to achieve high resolution and low line width roughness (LWR), meeting process requirements below 7 nm. Developing PACs with broad spectral compatibility to reduce R&D costs for photoresist manufacturers; creating PACs compatible with aqueous development to minimize organic solvent usage, optimize synthesis processes, and lower production costs for high-purity PACs. Key breakthroughs will focus on low-absorption, high-quantum-efficiency organometallic PACs to resolve the sensitivity-resolution trade-off in EUV photoresists.
This is the first one.
Contact us to learn more about our advanced electronic chemicals and speciality polymer materials, and how they can enhance your production performances.